ATP synthases - assembly and stability
Adenosine triphosphate (ATP) is the major energy source in biological systems and it is used for
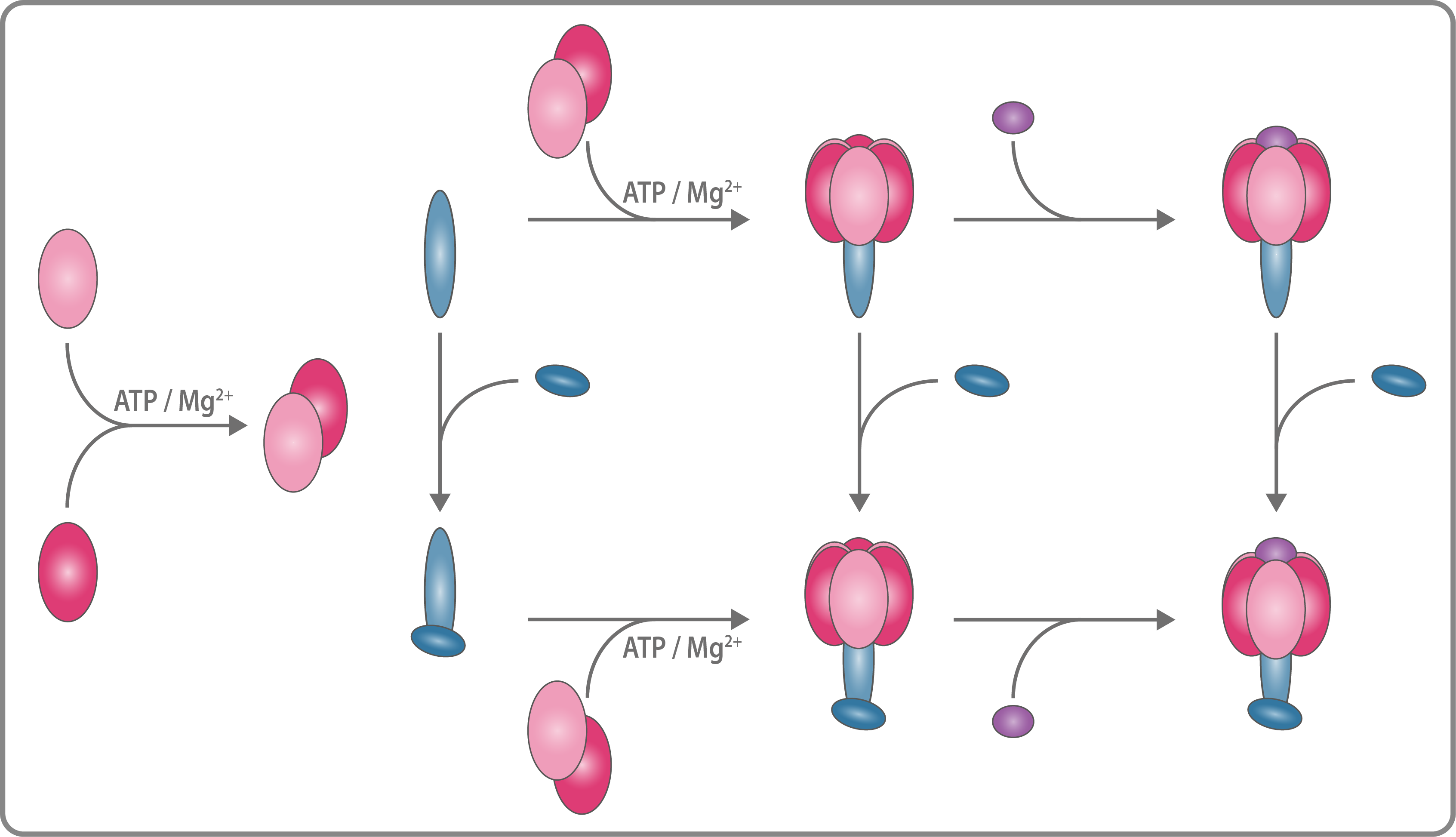
numerous energy-consuming reactions. The ATP synthesis is catalyzed by the ATP synthase, which uses a proton gradient across the cell membrane and converts it into chemical energy. ATP synthases are large multiprotein complexes (550-1600 kDa) which consist of a membrane embedded and a soluble subcomplex. We are interested in the assembly pathway of those complexes to
an intact ATP synthase. Using laser induced liquid bead ion desorption (LILBID) mass
spectrometry, we investigate important assembly intermediates and identify assembly requirements
for the formation of a ATP synthase.
As there are numerous human diseases associated with mutations in mitochondrial
ATP synthases, there are many unanswered questions regarding the effects of these mutations. Our goal is to obtain a comprehensive understanding of healthy mitochondrial ATP synthases and investigate ATP synthases with mutations known to cause neuropathological diseases. We are interested in the influence of those mutations towards complex stability, ATP binding efficiency and dimerization ability.
numerous energy-consuming reactions. The ATP synthesis is catalyzed by the ATP synthase, which uses a proton gradient across the cell membrane and converts it into chemical energy. ATP synthases are large multiprotein complexes (550-1600 kDa) which consist of a membrane embedded and a soluble subcomplex. We are interested in the assembly pathway of those complexes to
an intact ATP synthase. Using laser induced liquid bead ion desorption (LILBID) mass
spectrometry, we investigate important assembly intermediates and identify assembly requirements
for the formation of a ATP synthase.
As there are numerous human diseases associated with mutations in mitochondrial
ATP synthases, there are many unanswered questions regarding the effects of these mutations. Our goal is to obtain a comprehensive understanding of healthy mitochondrial ATP synthases and investigate ATP synthases with mutations known to cause neuropathological diseases. We are interested in the influence of those mutations towards complex stability, ATP binding efficiency and dimerization ability.
