heat shock proteins
Heat
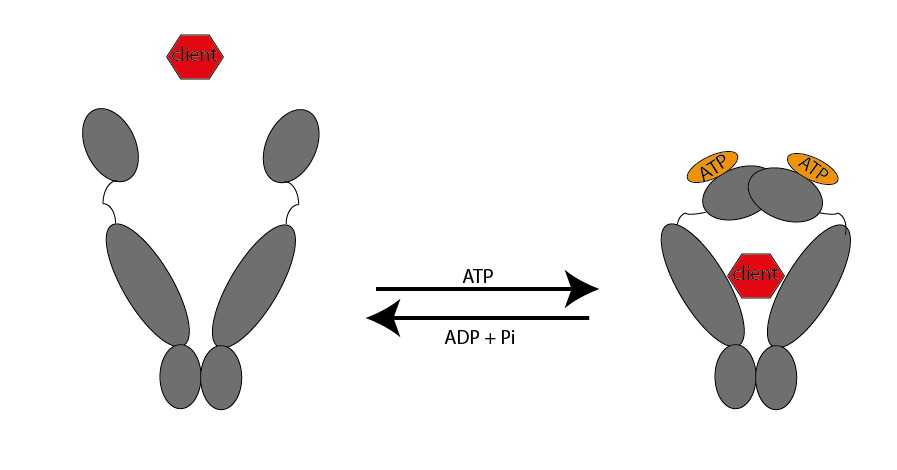
shock proteins are important chaperones that have distinctive
protective properties, such as preventing unwanted aggregation of
proteins or stabilizing and even (re-)folding its client molecules.
These proteins are abundant in almost all known organisms and play a
key role in stress response, as they are expressed when a cell is under
strain. Large parts of the human proteome are known to be client
molecules of one or another Heat shock protein. Our main target of
interest is the HSP90. Together with some co-chaperones it has an
important role in the lifecycle of glucocorticoid receptors and protein
kinases. We aim to describe the interactions between HSP90, its
co-chaperons and its client molecules via native mass spectrometry.
With regard to binding affinities and conformational changes, we try to
clarify parts of their vital role in the lifespan of cells.