photoreceptors
Photoreceptors are a widespread class of chromophore containing
proteins, which mediate several biological processes. In general, their
function is triggered by the absorption of light of species specific
wave length. The particular absorption maximum is determined by the
respective chromophore.
In general, after photoexcitation, either intramolecular proton or electron transfer mediate a conformational rearrangement of the photoreceptor, which is prerequisite for the recruitment of signaling partners of the downstream signaling process.
Ion Mobility Spectrometry (IMS) coupled to nano Electrospray Ionisation Mass Spectrometry (nESI-MS) is a powerful tool to study conformational protein dynamics. Currently, we are focusing on the time dependent analysis of structural rearrangement of photoactive proteins upon irradiation.
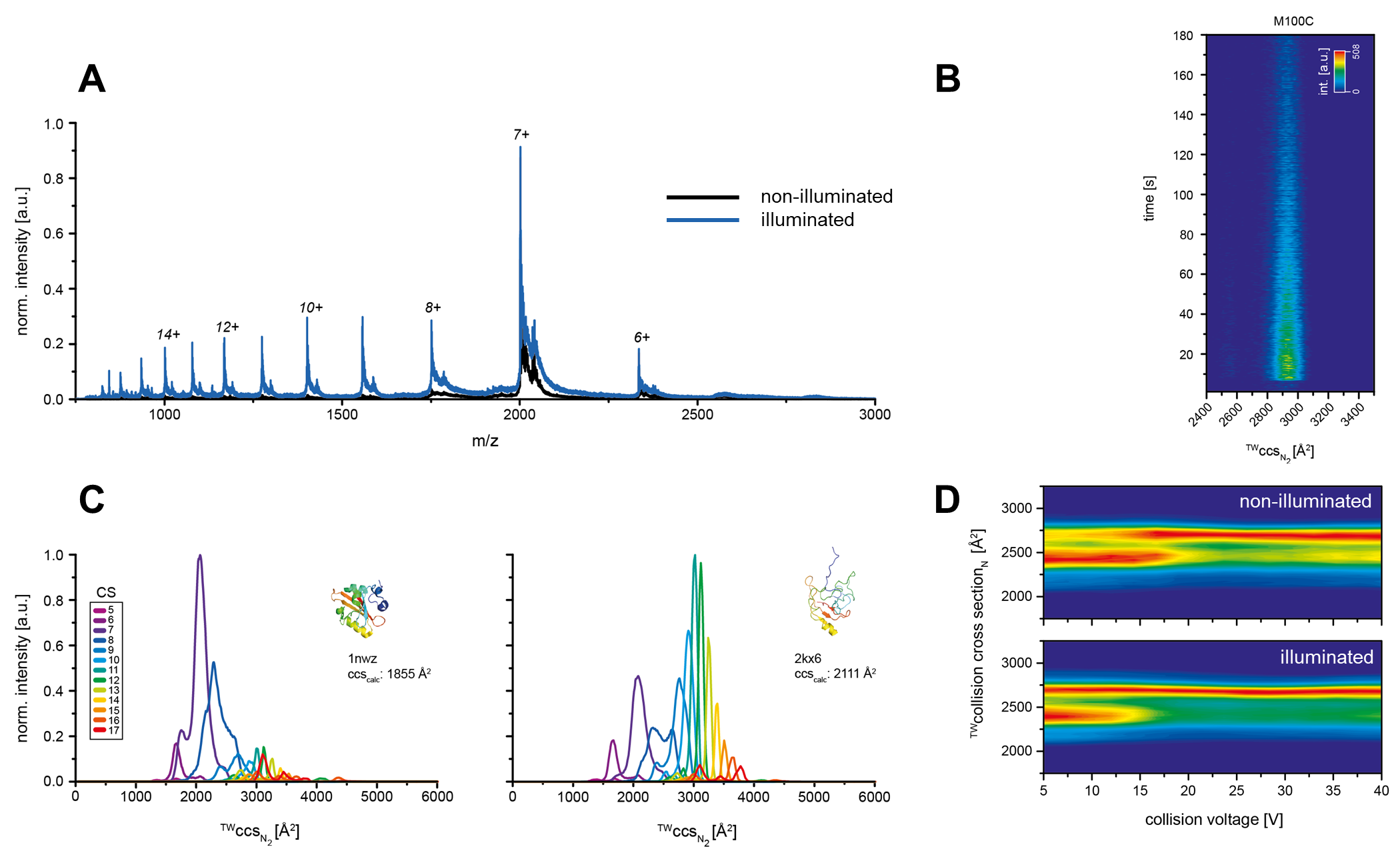
For this approach, we implemented a light source which allows sample irradiation during ionization. In case of light triggered transitions into a different state we then observe indicative changes in the spectra, such as additional m/z signals, corresponding to the respective signaling state conformation (fig. 1A).
Furthermore, IMS is a suitable technique to investigate light induced protein conformational changes on a time scale of ms to s to derive rate constants (fig. 1B). The obtained experimental collision cross sections (ccs) of the different protein conformations can then be used to correlate these with calculated ccs, based on NMR/crystal structures (fig. 1C). Additionally, collision induced unfolding experiments can be derived to obtain information about protein stability, which can be used to correlate these with conformational properties of the respective protein (fig. 1D).
In general, after photoexcitation, either intramolecular proton or electron transfer mediate a conformational rearrangement of the photoreceptor, which is prerequisite for the recruitment of signaling partners of the downstream signaling process.
Ion Mobility Spectrometry (IMS) coupled to nano Electrospray Ionisation Mass Spectrometry (nESI-MS) is a powerful tool to study conformational protein dynamics. Currently, we are focusing on the time dependent analysis of structural rearrangement of photoactive proteins upon irradiation.
For this approach, we implemented a light source which allows sample irradiation during ionization. In case of light triggered transitions into a different state we then observe indicative changes in the spectra, such as additional m/z signals, corresponding to the respective signaling state conformation (fig. 1A).
Furthermore, IMS is a suitable technique to investigate light induced protein conformational changes on a time scale of ms to s to derive rate constants (fig. 1B). The obtained experimental collision cross sections (ccs) of the different protein conformations can then be used to correlate these with calculated ccs, based on NMR/crystal structures (fig. 1C). Additionally, collision induced unfolding experiments can be derived to obtain information about protein stability, which can be used to correlate these with conformational properties of the respective protein (fig. 1D).